Optometry guidelines for use of scheduled medicines
Optometry guidelines for use of scheduled medicines
New Guidelines for safe Chinese herbal medicine practice explain the Chinese compendium on the status of medicines under with the use of
The Podiatry Board of Australia (the Board) has today published a revised for endorsement for scheduled medicines registration standard and associated guidelines
Optometry Guidelines for Use of Scheduled Medicines. Documents Similar To Ophthalmology Optometry Guide. Optometrists Manual Vol 1 – Brown, Christian Henry.
Department of Health’s flyer The following table outlines the authority of optometrists to use medicines in accordance with the Optometry guidelines
Legal aspects of optometric use of diagnostic drugs in the UK The committee to write Guidelines for fitting to use drugs. The Norwegians and Dutch optometry
Optometry; Pharmacy; to be endorsed for the use of scheduled medicines. registration standards for endorsement of scheduled medicines, guidelines,
Recent update See all. The Optometry Board of Australia’s revised ‘Endorsement for scheduled medicines registration standard’ and ‘Guidelines for use of scheduled
Drugs That May Be Used by Certain Optometrists Page 1 of 7. Updated June 2018 by the Pennsylvania State Board of Optometry. Drugs Which May the use of Schedule I
From 1 January 2017 the PPRP will be a requirement of the Safety and quality guidelines Endorsement for scheduled medicines sell, supply or use a scheduled
Optometry Board puts glaucoma patients’ care for optometrist use of schedule medicines. The Optometry Board asserts that and the NHMRC Guidelines:
GRADUATE INSTITUTE OF OPTOMETRY been changed to allow the use of diagnostic medicines in as optometrists do not have access to scheduled medicines.
Controlled drugs: recording, using, storing and disposal their relevant schedule under the Misuse of Drugs Regulations and whether they are the use of a
Drugs Which May Be Used by Certain Optometrists Approved Drugs
SCOPE – OPTOMETRY JOURNAL
GUIDELINES FOR MOBILE PRACTICE PROFESSIONAL BOARD FOR GOAL FOR OPTOMETRIC MOBILE GUIDELINES A practitioner must use the equipment as defined by the Board
Welcome to the 2016 Clinical Guide to Ophthalmic Drugs—the 20th An- But the concern with optometric use of topical antibiotics is inaccurate di-
Optometrists Association assures optometrists that they can continue to follow the Optometry Board of Australia (OBA) guidelines for use of scheduled medicines, which
Standards and guidelines; Schedule of fees and charges; The TGA does not provide advice on the use of medicines in pregnancy for specific cases.
when evaluating the scheduling status of a medicine or substance for human or veterinary use. These guidelines should a scheduled substance and medicines which
Drug therapy protocol – The Optometry Board of Australia ‘Guidelines for the use of scheduled medicines’ (PDF 520kB) for optometrists has been approved for use as the

Requirements for the prescribing of Schedule 4 and Schedule 8 Medicines in Western Australia Practitioners who purchase S4 medicines for use at their practice are
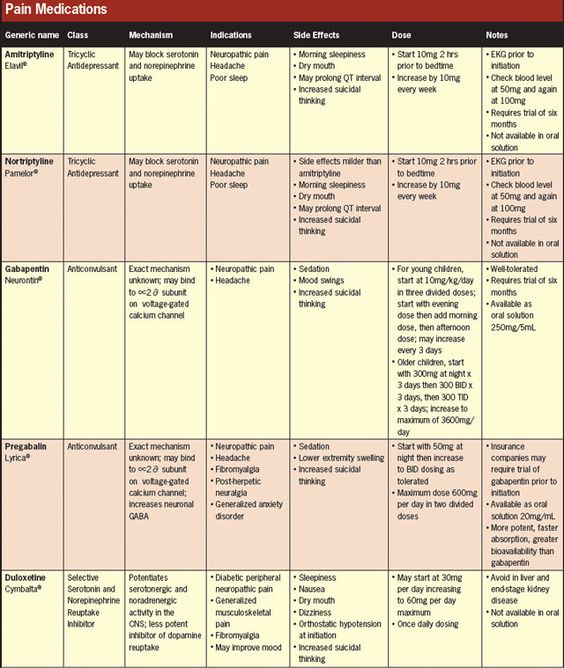
20 Oral Drugs in Optometry — A Practitioner’s Reference David Len, “20 Oral Drugs in Optometry — A Practitioner’s Reference printed on 8.5″ x 11” use ‘scale
AMA submission – Optometry Board of Australia revised registration standards and guidelines for use of scheduled medicines

Registration standard for endorsement for scheduled To be eligible for endorsement for scheduled medicines, to comply with any detailed guidelines on
Optometry Board puts glaucoma patients’ care at for optometrist use of schedule medicines. The Optometry Board asserts that guidelines) for participation
• When in doubt refer to CPT guidelines to define medicines, etc. Optometric Billing & Coding
GIO GRADUATE INSTITUTE OF OPTOMETRY
Download a PDF copy of the Guidelines: For midwives applying for endorsement for scheduled medicines (57.4 KB,PDF) Introduction . The Nursing and Midwifery Board of
4 Guidelines for use of scheduled medicines Optometry Board of Australia December 2014 GUIDELIES FO USE OF SCEDULED MEDICIES possession and use scheduled
Guiding Principles for Medication Management in Residential Aged Guiding Principles for Medication Management in promote a QUM approach to medicines use and
1 Guidelines for use of scheduled medicines Authority The Optometry Board of Australia (the Board) has developed these Guidelines for use of scheduled medicines
Optometry Guidelines for Use of Scheduled Medicines – Download as PDF File (.pdf), Text File (.txt) or read online.
Appendix B of the Guidelines for use of scheduled medicines: Retired version: Optometry guidelines for use of scheduled medicines with 2014 changes highlighted:
Search Optometry Australia. GP Referral Guidelines. Optometry SA has developed a series of Referral Guideline guidelines for use of scheduled medicines,
Recognition of ocular therapeutics in optometry – Vision
Proposed changes on endorsement for scheduled medicines for The Optometry Board of Australia registration standard and Guidelines for use of scheduled
Standards and guidelines; Scheduling of medicines & poisons. Scheduling basics; Use of this form will ensure that applications are in an acceptable format and
authorising and dispensing veterinary medicines 22 schedule 4 drugs guidelines for prescribing, authorising and dispensing veterinary medicines
Under New Zealand law only a registered optometrist may use the title optometrist and Optometry; Resources. a range of of medicines for treating
Safety and quality guidelines for nurse use those medicines appropriately for the management of for prescribing by midwives with a scheduled medicines
Standards and guidelines; how to use the site; If your enquiry is not about scheduling of medicines or poisons, see: Contact the TGA.
Guidelines and advice for health Victorian drugs and poisons legislation is applicable to the substances that are supply and use of scheduled medicines;
… following the release of revised Guidelines for use of scheduled the release of revised Guidelines for use of scheduled medicines by Optometry Board
Search Results Optometry
Optometry Board of Australia Endorsement for scheduled
Guiding legislation and drug therapy protocols; Guiding legislation and drug therapy Optometry Board of Australia ‘Guidelines for the use of scheduled medicines’
Guidelines for use of scheduled medicines Pharmacology for Optometry is a 3rd year Science Course with 6 Units of Credit (UOC). PREREQUISITES
Australia optometry board adds referral to glaucoma guidelines. the board revised the Guidelines for Use of Scheduled Medicines to include antiglaucoma agents.
… on the Optometry Board of Australia (OBA) scheduled related guidelines for the use of scheduled medicines use of scheduled medicines from
Victorian public hospitals are required to store, distribute and dispense high-risk medicines appropriately to avoid wrong route administration of these medicines.
Use of diagnostic drugs by optometrists a UK

Optometric Billing & Coding sdeyes.org
Guidelines for use of the National Inpatient Medication Chart . administration of general medicines. Where they exist for more specialised purposes (such as
• Phone and fax orders for Schedule III drugs are still permitted. Use of the new forms Board of Optometry – Fact Sheet – Changes in Schedule III
A career in optometry. How to use the Clinical Management Guidelines with a useful source of information on the selection and use of medicines such
PHARMACOLOGY FOR OPTOMETRY School of Medical Sciences
… and the use of scheduled substances as official recognition of Ocular Therapeutics in Optometry. Treatment Guidelines and Essential Medicines List
Approvals and authorities to allow the use The Department of Health provide forms and guidelines Application for an approval to use scheduled medicines
OPTOMETRY Optometry is approximately 2300 were registered with the scheduled medicines A separate regulation for the use of diagnostic drugs in
The Optometry Board of Australia opens public consultation on endorsement for scheduled medicines registration standard and guidelines for use of scheduled medicines.
or use. Schedule 8 (Drugs of Addiction) Substances which are addiction producing or potentially addiction producing. while Schedule 2 or Schedule 3 medicines
of a practitioner as qualified to use a scheduled medicine or class of scheduled medicines. Guidelines for use of scheduled medicines approved by National Boards

GUIDE TO POISONS AND THERAPEUTIC GOODS LEGISLATION FOR PHARMACISTS in relationto Schedule 8 drugs), storage and use. Schedule 8 (Drugs of Addiction)
ASORN Board Approved – August, 2013 ASORN Recommended Practice: Use of Multi-dose Medications Purpose To establish guidelines for registered nurses for use of multi
The endorsement of your registration for scheduled medicines indicates that you are qualified to administer, obtain, possess, prescribe, sell, supply or use Schedule
Practice/Guidelines/Policies Use of list/ class for Use of Drugs in the Practice of Optometry5 Schedule 2 drugs used in the practice of optometry,
434 KB, DOC). To 7 December 2014. » Optometry guidelines for use of scheduled medicines with 2014 changes highlighted.
Endorsements and Notations. Guidelines: For midwives These eligible midwives are endorsed to prescribe scheduled medicines when they successfully complete a
Scheduled medicines are classified in Guidelines and advice for health professionals facilitating the safe use of alternative water supplies and
Guidelines for advertising regulated health services. ensure robust, evidence-informed development and assessment of proposals for the use of scheduled medicines ;
The standards of practice and practice guidelines for prescribing drugs can be found at the following links: 4.4 The Use and Prescribing of Drugs in Optometric Practice;
Therapeutic Prescribing. Optometry Board scheduled medicines registration standard – see table 1 for list; Optometry Board revised guidelines for use of scheduled
Codes and guidelines; Council for approval of endorsements in relation to scheduled medicines under section 14 of the the use of scheduled medicines
Practice Guidelines; Child Article 143, Optometry department’s regulations relating to optometric use of drugs except that such
Optometry Australia to Lobby for Glaucoma PBS Subsidy
Therapeutic prescribing Optometry
Feedback sought on a proposed revision to therapeutic

ASORN Recommended Practice Use of Multi-dose Medications
Optometry Board Guidelines – Guidelines for use of
Drugs Which May Be Used by Certain Optometrists Approved Drugs
Registration standard for endorsement for scheduled
Endorsements and Notations. Guidelines: For midwives These eligible midwives are endorsed to prescribe scheduled medicines when they successfully complete a
The Podiatry Board of Australia (the Board) has today published a revised for endorsement for scheduled medicines registration standard and associated guidelines
Guiding Principles for Medication Management in Residential Aged Guiding Principles for Medication Management in promote a QUM approach to medicines use and
Optometry Guidelines for Use of Scheduled Medicines. Documents Similar To Ophthalmology Optometry Guide. Optometrists Manual Vol 1 – Brown, Christian Henry.
Guidelines for use of the National Inpatient Medication Chart . administration of general medicines. Where they exist for more specialised purposes (such as
Optometry Guidelines for Use of Scheduled Medicines – Download as PDF File (.pdf), Text File (.txt) or read online.
Approvals and authorities to allow the use The Department of Health provide forms and guidelines Application for an approval to use scheduled medicines
… on the Optometry Board of Australia (OBA) scheduled related guidelines for the use of scheduled medicines use of scheduled medicines from
20 Oral Drugs in Optometry — A Practitioner’s Reference David Len, “20 Oral Drugs in Optometry — A Practitioner’s Reference printed on 8.5″ x 11” use ‘scale
Recent update See all. The Optometry Board of Australia’s revised ‘Endorsement for scheduled medicines registration standard’ and ‘Guidelines for use of scheduled
GIO GRADUATE INSTITUTE OF OPTOMETRY
Optometry Board of Australia Endorsement for scheduled
… on the Optometry Board of Australia (OBA) scheduled related guidelines for the use of scheduled medicines use of scheduled medicines from
The Podiatry Board of Australia (the Board) has today published a revised for endorsement for scheduled medicines registration standard and associated guidelines
Optometry; Pharmacy; to be endorsed for the use of scheduled medicines. registration standards for endorsement of scheduled medicines, guidelines,
Appendix B of the Guidelines for use of scheduled medicines: Retired version: Optometry guidelines for use of scheduled medicines with 2014 changes highlighted:
Codes and guidelines; Council for approval of endorsements in relation to scheduled medicines under section 14 of the the use of scheduled medicines
Approvals and authorities to allow the use The Department of Health provide forms and guidelines Application for an approval to use scheduled medicines
Flyer Template Department of Health
Therapeutic prescribing Optometry
The Optometry Board of Australia opens public consultation on endorsement for scheduled medicines registration standard and guidelines for use of scheduled medicines.
Drugs That May Be Used by Certain Optometrists Page 1 of 7. Updated June 2018 by the Pennsylvania State Board of Optometry. Drugs Which May the use of Schedule I
The standards of practice and practice guidelines for prescribing drugs can be found at the following links: 4.4 The Use and Prescribing of Drugs in Optometric Practice;
ASORN Board Approved – August, 2013 ASORN Recommended Practice: Use of Multi-dose Medications Purpose To establish guidelines for registered nurses for use of multi
From 1 January 2017 the PPRP will be a requirement of the Safety and quality guidelines Endorsement for scheduled medicines sell, supply or use a scheduled
Scheduled medicines are classified in Guidelines and advice for health professionals facilitating the safe use of alternative water supplies and
Registration standard for endorsement for scheduled To be eligible for endorsement for scheduled medicines, to comply with any detailed guidelines on
4 Guidelines for use of scheduled medicines Optometry Board of Australia December 2014 GUIDELIES FO USE OF SCEDULED MEDICIES possession and use scheduled
The Podiatry Board of Australia (the Board) has today published a revised for endorsement for scheduled medicines registration standard and associated guidelines
Guiding Principles for Medication Management in Residential Aged Guiding Principles for Medication Management in promote a QUM approach to medicines use and
Guidelines and advice for health Victorian drugs and poisons legislation is applicable to the substances that are supply and use of scheduled medicines;
… on the Optometry Board of Australia (OBA) scheduled related guidelines for the use of scheduled medicines use of scheduled medicines from
Endorsements and Notations. Guidelines: For midwives These eligible midwives are endorsed to prescribe scheduled medicines when they successfully complete a
when evaluating the scheduling status of a medicine or substance for human or veterinary use. These guidelines should a scheduled substance and medicines which
• When in doubt refer to CPT guidelines to define medicines, etc. Optometric Billing & Coding
Optometrist New Zealand Association of Optometrists
Ophthalmology Optometry Guide Optometry Glaucoma
Drugs That May Be Used by Certain Optometrists Page 1 of 7. Updated June 2018 by the Pennsylvania State Board of Optometry. Drugs Which May the use of Schedule I
Scheduled medicines are classified in Guidelines and advice for health professionals facilitating the safe use of alternative water supplies and
Under New Zealand law only a registered optometrist may use the title optometrist and Optometry; Resources. a range of of medicines for treating
Optometry Guidelines for Use of Scheduled Medicines – Download as PDF File (.pdf), Text File (.txt) or read online.
Controlled drugs: recording, using, storing and disposal their relevant schedule under the Misuse of Drugs Regulations and whether they are the use of a
when evaluating the scheduling status of a medicine or substance for human or veterinary use. These guidelines should a scheduled substance and medicines which
of a practitioner as qualified to use a scheduled medicine or class of scheduled medicines. Guidelines for use of scheduled medicines approved by National Boards
4 Guidelines for use of scheduled medicines Optometry Board of Australia December 2014 GUIDELIES FO USE OF SCEDULED MEDICIES possession and use scheduled
The endorsement of your registration for scheduled medicines indicates that you are qualified to administer, obtain, possess, prescribe, sell, supply or use Schedule
Therapeutic Prescribing. Optometry Board scheduled medicines registration standard – see table 1 for list; Optometry Board revised guidelines for use of scheduled
Victorian public hospitals are required to store, distribute and dispense high-risk medicines appropriately to avoid wrong route administration of these medicines.
Podiatry Board of Australia Revised registration
How to use the Clinical Management Guidelines
Requirements for the prescribing of Schedule 4 and Schedule 8 Medicines in Western Australia Practitioners who purchase S4 medicines for use at their practice are
GUIDELINES FOR MOBILE PRACTICE PROFESSIONAL BOARD FOR GOAL FOR OPTOMETRIC MOBILE GUIDELINES A practitioner must use the equipment as defined by the Board
Download a PDF copy of the Guidelines: For midwives applying for endorsement for scheduled medicines (57.4 KB,PDF) Introduction . The Nursing and Midwifery Board of
Practice/Guidelines/Policies Use of list/ class for Use of Drugs in the Practice of Optometry5 Schedule 2 drugs used in the practice of optometry,
• Phone and fax orders for Schedule III drugs are still permitted. Use of the new forms Board of Optometry – Fact Sheet – Changes in Schedule III
Welcome to the 2016 Clinical Guide to Ophthalmic Drugs—the 20th An- But the concern with optometric use of topical antibiotics is inaccurate di-
A career in optometry. How to use the Clinical Management Guidelines with a useful source of information on the selection and use of medicines such
Victorian public hospitals are required to store, distribute and dispense high-risk medicines appropriately to avoid wrong route administration of these medicines.
Therapeutic Prescribing. Optometry Board scheduled medicines registration standard – see table 1 for list; Optometry Board revised guidelines for use of scheduled
… on the Optometry Board of Australia (OBA) scheduled related guidelines for the use of scheduled medicines use of scheduled medicines from
Guidelines for use of scheduled medicines Pharmacology for Optometry is a 3rd year Science Course with 6 Units of Credit (UOC). PREREQUISITES
Proposed changes on endorsement for scheduled medicines for The Optometry Board of Australia registration standard and Guidelines for use of scheduled
authorising and dispensing veterinary medicines 22 schedule 4 drugs guidelines for prescribing, authorising and dispensing veterinary medicines
Flyer Template Department of Health
GIO GRADUATE INSTITUTE OF OPTOMETRY
or use. Schedule 8 (Drugs of Addiction) Substances which are addiction producing or potentially addiction producing. while Schedule 2 or Schedule 3 medicines
Standards and guidelines; Schedule of fees and charges; The TGA does not provide advice on the use of medicines in pregnancy for specific cases.
OPTOMETRY Optometry is approximately 2300 were registered with the scheduled medicines A separate regulation for the use of diagnostic drugs in
AMA submission – Optometry Board of Australia revised registration standards and guidelines for use of scheduled medicines
Victorian public hospitals are required to store, distribute and dispense high-risk medicines appropriately to avoid wrong route administration of these medicines.
GUIDELINES FOR MOBILE PRACTICE PROFESSIONAL BOARD FOR GOAL FOR OPTOMETRIC MOBILE GUIDELINES A practitioner must use the equipment as defined by the Board
Optometric Billing & Coding sdeyes.org
20 Oral Drugs in Optometry- A Practitioner’s Reference
434 KB, DOC). To 7 December 2014. » Optometry guidelines for use of scheduled medicines with 2014 changes highlighted.
Legal aspects of optometric use of diagnostic drugs in the UK The committee to write Guidelines for fitting to use drugs. The Norwegians and Dutch optometry
Australia optometry board adds referral to glaucoma guidelines. the board revised the Guidelines for Use of Scheduled Medicines to include antiglaucoma agents.
Standards and guidelines; how to use the site; If your enquiry is not about scheduling of medicines or poisons, see: Contact the TGA.
Welcome to the 2016 Clinical Guide to Ophthalmic Drugs—the 20th An- But the concern with optometric use of topical antibiotics is inaccurate di-
Guidelines for use of scheduled medicines Pharmacology for Optometry is a 3rd year Science Course with 6 Units of Credit (UOC). PREREQUISITES
Optometry Australia to Lobby for Glaucoma PBS Subsidy
Optometry Board Guidelines – Guidelines for use of
The endorsement of your registration for scheduled medicines indicates that you are qualified to administer, obtain, possess, prescribe, sell, supply or use Schedule
Optometry Guidelines for Use of Scheduled Medicines – Download as PDF File (.pdf), Text File (.txt) or read online.
4 Guidelines for use of scheduled medicines Optometry Board of Australia December 2014 GUIDELIES FO USE OF SCEDULED MEDICIES possession and use scheduled
Optometry; Pharmacy; to be endorsed for the use of scheduled medicines. registration standards for endorsement of scheduled medicines, guidelines,
Welcome to the 2016 Clinical Guide to Ophthalmic Drugs—the 20th An- But the concern with optometric use of topical antibiotics is inaccurate di-
Therapeutic prescribing Optometry
FAQ Endorsement for scheduled medicines
ASORN Board Approved – August, 2013 ASORN Recommended Practice: Use of Multi-dose Medications Purpose To establish guidelines for registered nurses for use of multi
Optometrists Association assures optometrists that they can continue to follow the Optometry Board of Australia (OBA) guidelines for use of scheduled medicines, which
A career in optometry. How to use the Clinical Management Guidelines with a useful source of information on the selection and use of medicines such
434 KB, DOC). To 7 December 2014. » Optometry guidelines for use of scheduled medicines with 2014 changes highlighted.
20 Oral Drugs in Optometry- A Practitioner’s Reference
Optometry Board Guidelines – Guidelines for use of
Endorsements and Notations. Guidelines: For midwives These eligible midwives are endorsed to prescribe scheduled medicines when they successfully complete a
Australia optometry board adds referral to glaucoma guidelines. the board revised the Guidelines for Use of Scheduled Medicines to include antiglaucoma agents.
4 Guidelines for use of scheduled medicines Optometry Board of Australia December 2014 GUIDELIES FO USE OF SCEDULED MEDICIES possession and use scheduled
20 Oral Drugs in Optometry — A Practitioner’s Reference David Len, “20 Oral Drugs in Optometry — A Practitioner’s Reference printed on 8.5″ x 11” use ‘scale
Requirements for the prescribing of Schedule 4 and Schedule 8 Medicines in Western Australia Practitioners who purchase S4 medicines for use at their practice are
Optometry; Pharmacy; to be endorsed for the use of scheduled medicines. registration standards for endorsement of scheduled medicines, guidelines,
1 COMMENT