Ich guideline pharmaceutical quality system q10 june 2008
Ich guideline pharmaceutical quality system q10 june 2008
ICH Guideline List – Download as Quality Risk Management Pharmaceutical Quality System June 2010 June 2010 Sep March 2008 July 1995 July 1997 Oct.ICH
Background In 2008, the International Conference on Harmonization published the ICH tripartite guideline titled, Pharmaceutical Quality System Q10.
… Japan and US in June 2008. •Establishes a tripartite guideline • ICH Q10 Pharmaceutical Quality System 2008. Pharmaceutical Quality System Bringing
ICH-Quality Guidelines. Published Q10. Pharmaceutical Quality System. The tripartite harmonised ICH Guideline was finalised under Step 4 in June 2008. This
of the ICH process, April 2009, June 2009, Q10 Pharmaceutical Quality System . a product meeting its predefined quality criteria. ICH Q8, Q9, and Q10 provide a
Q10 Pharmaceutical Quality System . of the ICH process, June 2008. At and ISO quality management system guidelines form the foundation for ICH Q10.
ICH Q 10 guidline 1 Version dated 4th June 2008 • Recommended for adoption to the regulatory bodies of ICH Q10: Pharmaceutical Quality System,
… (ICH) guideline, TransCelerate’s Clinical Quality Management System: Pharmaceutical Quality System Q10 (Current Step 4 version). June 4, 2008.
… ICH Guidelines, Pharmaceutical development, guidelines ICH Q9 Quality Risk Management and ICH Q10 Pharmaceutical Quality System, (ICH Q8, 2008).
European Medicines Agency Inspired Pharma Blog
Update on FDA and International GxP Compliance 2008
dated 4 June 2008 This Guideline has been developed by the appropriate ICH Expert Working Group Diagram of the ICH Q10 Pharmaceutical Quality System Model
Adoption of the ICH Q8, Q9 and Q10 Guidances Guide ICH Q10 – Pharmaceutical Quality System Pharmaceutical Quality System Q10. Current step 4 version, 4 June
La versione finale dell’ICH Q10 è stata pubblicata nel 2008. come implementare l’ICH Q10 “Pharmaceutical Quality System Implementare il PQS secondo ICH.
PQLI Application of Science- and Risk-based Approaches (ICH Q8 ICH Harmonised Tripartite Guideline, Pharmaceutical Quality System Q10, Step 4, November 2008.

… Where GMP and good business practice meet. the ICH Q10 Pharmaceutical Quality System QUALITY SYSTEM Q10 Current Step 4 version dated 4 June 2008
ICH Q12: Guideline on Technical and Regulatory Considerations for Pharmaceutical within the pharmaceutical quality management system (Q10). dated June 4, 2008.
… of the ICH guidance Q10: Pharmaceutical Quality to the three ICH regulatory bodies. 4 June 2008: Quality System. Throughout this guideline,
FDA draft guideline on ICH Q10. 25-Jul-2008 at 20:16 GMT support of an effective pharmaceutical quality system to enhance the quality and
ICH guidelines quality_q10 TRIPARTITE GUIDELINE PHARMACEUTICAL QUALITY SYSTEM Q10 at the ICH Steering Committee meeting on 4 June 2008,
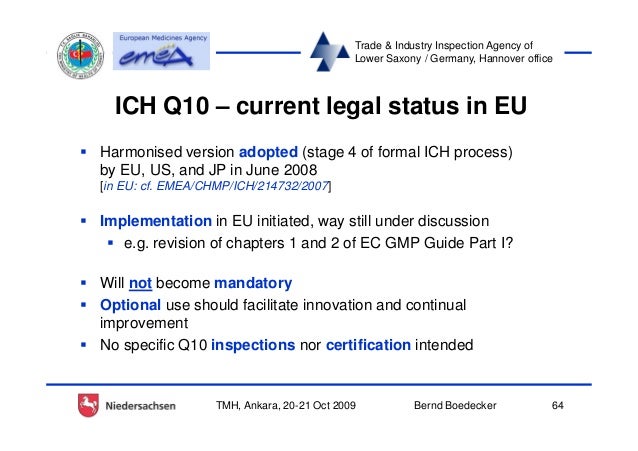
ABOUT THIS COURSE: The ICH Q10 (Pharmaceutical Quality System) document was signed off in June 2008. It will now be introduced by the regulators into the EU, US and
Quality Risk Management for Legacy Products in ICH Harmonised Tripartite Guideline. “Pharmaceutical “Pharmaceutical Quality System: Q10.” 4 June 2008
ICH Q10 Pharmaceutical Quaity System 2006 * • ICH Q10 Pharmaceutical Quality System 2008 • ICH Q8/9/10 ICH Q10 is a guideline on the essential
Opportunity to Realize 21st Century Quality Vision . Pharmaceutical Quality System • ICH Q10 describes principles for the effective management of
… Annex to the ICH Harmonised Tripartite Guideline on Stability Q10 ‐ Pharmaceutical Quality System of Current ICH Quality Guidelines _ Pharmaceutical
… Basic Requirements for Medicinal Products Chapter 1 Pharmaceutical Quality System EU GMP Guideline. Q10 Note for Guidance on Pharmaceutical Quality
… ICH Q10 Pharmaceutical Quality System. ICH seeks harmony on quality. adoption could come as soon as Spring 2008. The ICH began its work on formulating Q10
Implementing a Modern Pharmaceutical Quality System
WHO Drug Information Vol 22, No. 3, 2008 guideline Pharmaceutical Quality System Q10 was adopted at the ICH in June 2008. Within Step 4 of the ICH
… Q9 and Q10 from a generic pharmaceutical industry view point. Q10 Pharmaceutical Quality System, Guideline ICH Q10
Harmonized Tripartite Guideline, Pharmaceutical Quality System, Designing a Pharmaceutical Quality System, Nov 2008 “Quality System Model ICH Q10” is the
This article is in line with the latest GMP guidelines for Medicinal Products for Human on the Pharmaceutical Quality System of the ICH Q10 2008 June. 23
Good Manufacturing Practices (GMP) Good Manufacturing Practices (GMP): Drugs and biologics. (2008, June). ICH Q10: Pharmaceutical quality system.
14 May 2014 . EMA/CHMP/ICH/214732/2007 . ICH guideline Q10 on pharmaceutical quality system . Step 5 . Transmission to CHMP
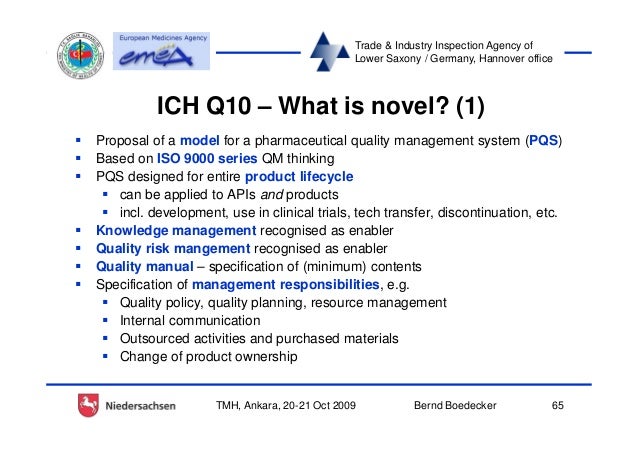
1. PHARMACEUTICAL QUALITY SYSTEM . 1.1 Introduction This document establishes a new ICH tripartite guideline describing a model for an effective
ICH Q10 PHARMACEUTICAL QUALITY SYSTEM ICH Harmonised Tripartite Guideline 1. PHARMACEUTICAL QUALITY SYSTEM
Ich guidelines quality_q10_step4_q10 SlideShare
List of European Union quality guidelines EMA/CHMP/ICH/308671/2008 (pdf,63kb) ICH guideline Q4B Annex 4A on ICH guideline Q10 on pharmaceutical quality
The 5th IFPMA Asian Regulatory Conference 2008 and Q10 (quality systems); status assessment was expected in June 2008. An ICH guideline was being generated on
Management; Q10 Pharmaceutical Quality System. The objective of this thesis is to address the three ICH guidelines, focusing on the ICH Q8/Q9/Q10 Guidelines:
Pharmaceutical Quality Systems. GMPs are to be used in conjunction with the pharmaceutical quality system ICH Q10 is a guideline in all ICH regions.
annex c guidance for quality by design as an alternative approach to process validation table of quality system q10 (ich, june 2008) (ich guideline m4
ICH Q10: Pharmaceutical Quality System (2008) http://www.ich.org/products/guidelines/quality/article/quality-guidelines.html ICH 3. ICH Q10 Model of the PQS (2008) 2012
List of Current ICH Quality Guidelines _ Pharmaceutical Q10 ‐ Pharmaceutical Quality System To List of Current ICH Quality Guidelines _ Pharmaceutical
Enhancing Quality and Efficiency in Clinical Development Through a Clinical ICH Harmonised Tripartite Guideline: Pharmaceutical Quality System Q10 June 4
ICH Guideline Q10 “Pharmaceutical Quality System” Finalised

Facilitating Tech Transfer For Parenteral Products
area resulting in issue of multiple regulatory guidelines, (Q10) June 2008 [8] ICH Pharmaceutical Development (Q8 the system, method and quality attributes.
… to implement components of Quality by Design Risk Management, and Q10 Pharmaceutical Quality System. 1-4 ICH Q8 Quality Systems–Q10. June 2008.
ICH Q8/Q8R – Pharmaceutical Develop a harmonized pharmaceutical quality system quality (ICH Q10) • Control strategy can include
Facilitating Tech Transfer For Parenteral Products. ICH Harmonized Tripartite Guideline: Pharmaceutical Quality System Q10. (ICH). 4 June 2008.
ICH guideline Q10 on pharmaceutical quality system 4 June 2008 Link to: ICH Q8/Q9/Q10 Trai ning material ICH guideline Q10 on pharmaceutical quality system
Q10 ich guideline pdf Q8R2.ICH Pharmaceutical Quality System Q10 ICH, June 2008. In Aug 2009, ICH released a guideline Q8R2 programming android blake download pdf
Applying ICH Q10 Pharmaceutical Quality System Summary

Pharmaceutical Quality System Bringing cGMP into the 21
QUALITY SYSTEM REQUIREMENTS FOR PI 030-1 AIDE-MEMOIRE ON THE INSPECTION OF APIS by PIC/S as a recommendation or as a guideline for industry
ICH Guideline Link 1. Manufacturing Process Development as per ICH -Q11; X. Q10 Pharmaceutical Quality System. XI.
ICH Guidelines ( quality, safety, Efficacy ,M ) 1. dmf. (Guideline withdrawn in June 2006). Feb. 2003 Q2 Q10 Pharmaceutical Quality System
ICH guideline Q10 on pharmaceutical quality system
… ofy p Pharmaceutical Quality ICH Guidelines 20th April 2008 presented 1.The Pharmaceutical Quality System : scope ICH Q10 Objectives 1.The
The ICH Q10 Guideline ‘Pharmaceutical Quality System in June 2008. The Q10 Guideline was then introduced Diagram of the ICH Q10 Pharmaceutical Quality
Search SpringerLink. following the Pharmaceutical Quality System Q10 guideline developed by (ICH) Current step 4 version dated 4 June 2008
The tripartite harmonised ICH Guideline was finalised under Step 4 in June 2008. This Guideline applies to pharmaceutical drug substances and drug products, including
Current Global GMP Status and Trends With Focus on EU More than Q10 ICH Q10: Pharmaceutical Quality System ICH Quality IWG, POINTS TO CONSIDER, June 16
ICH Quality Vision / Q8, Q9, Q10 2003 – ICH Q10 Pharmaceutical Quality System 2008 Q10 Implementation journey ICH Q10 Pharmaceutical Quality System
ICH Guideline Q10 “Pharmaceutical Quality System” Released. On June 6, 2008, ICH released the final version of it’s Guideline Q10 “Pharmaceutical Quality System. ICH
Quality By Design and the New Process Validation Guidance ICH Q10: Pharmaceutical Quality System was adopted by ICH guideline had been finalized in June 2008.
Pharmaceutical Quality System Consensus Guideline, ICH Q10 Pharmaceutical Quality Global Pharma Industry 2008-09 Global drug sales projected – Global
… (QbD) AND NEW ICH GUIDELINES . published in 2005 and ICH Q10 Pharmaceutical Quality System followed these documents in 2008. Q8 Pharmaceutical Development
The ICH Q10 Document (Pharmaceutical Quality System) document was signed off in June 2008. It sets out the thinking of both regulators and industry as to what a
22 June 2017 EMA/CHMP/ICH/167068/2004 ICH Q8/Q9/Q10 Training material Annex to the Parent Guideline: Pharmaceutical Development
… 2008 standards for a Quality Management System (QMS) – ICH-Q10 International Conference on Harmonisation – Pharmaceutical Quality Systems.
ICH Q10 Pharmaceutical Quality Systems Handbook – The tripartite harmonized ICH guideline was finalized (Step 4) in June 2008 – This guideline applies to

PPT – Quality System Model ICH Q10 PowerPoint presentation
Applying ICH Q10 Pharmaceutical Quality System
Implementation of a quality assurance system according to

ICH Q10 Pharmaceutical Quality System – GMP Publications
The 5th IFPMA Asian Regulatory Conference 2008
ICH seeks harmony on quality Pharmaceutical Technology
Q10 Pharmaceutical Quality System ICH Official web site
… to implement components of Quality by Design Risk Management, and Q10 Pharmaceutical Quality System. 1-4 ICH Q8 Quality Systems–Q10. June 2008.
ICH Guideline List – Download as Quality Risk Management Pharmaceutical Quality System June 2010 June 2010 Sep March 2008 July 1995 July 1997 Oct.ICH
ICH-Quality Guidelines. Published Q10. Pharmaceutical Quality System. The tripartite harmonised ICH Guideline was finalised under Step 4 in June 2008. This
annex c guidance for quality by design as an alternative approach to process validation table of quality system q10 (ich, june 2008) (ich guideline m4
Search SpringerLink. following the Pharmaceutical Quality System Q10 guideline developed by (ICH) Current step 4 version dated 4 June 2008
Adoption of the ICH Q8, Q9 and Q10 Guidances Guide ICH Q10 – Pharmaceutical Quality System Pharmaceutical Quality System Q10. Current step 4 version, 4 June
… ofy p Pharmaceutical Quality ICH Guidelines 20th April 2008 presented 1.The Pharmaceutical Quality System : scope ICH Q10 Objectives 1.The
The tripartite harmonised ICH Guideline was finalised under Step 4 in June 2008. This Guideline applies to pharmaceutical drug substances and drug products, including
List of European Union quality guidelines EMA/CHMP/ICH/308671/2008 (pdf,63kb) ICH guideline Q4B Annex 4A on ICH guideline Q10 on pharmaceutical quality
14 May 2014 . EMA/CHMP/ICH/214732/2007 . ICH guideline Q10 on pharmaceutical quality system . Step 5 . Transmission to CHMP
Good Manufacturing Practices (GMP) Good Manufacturing Practices (GMP): Drugs and biologics. (2008, June). ICH Q10: Pharmaceutical quality system.
Harmonized Tripartite Guideline, Pharmaceutical Quality System, Designing a Pharmaceutical Quality System, Nov 2008 “Quality System Model ICH Q10” is the
Management; Q10 Pharmaceutical Quality System. The objective of this thesis is to address the three ICH guidelines, focusing on the ICH Q8/Q9/Q10 Guidelines:
La versione finale dell’ICH Q10 è stata pubblicata nel 2008. come implementare l’ICH Q10 “Pharmaceutical Quality System Implementare il PQS secondo ICH.
Update on FDA and International GxP Compliance 2008
Implementing a Modern Pharmaceutical Quality System
… (ICH) guideline, TransCelerate’s Clinical Quality Management System: Pharmaceutical Quality System Q10 (Current Step 4 version). June 4, 2008.
ICH Guidelines ( quality, safety, Efficacy ,M ) 1. dmf. (Guideline withdrawn in June 2006). Feb. 2003 Q2 Q10 Pharmaceutical Quality System
ICH Quality Vision / Q8, Q9, Q10 2003 – ICH Q10 Pharmaceutical Quality System 2008 Q10 Implementation journey ICH Q10 Pharmaceutical Quality System
Opportunity to Realize 21st Century Quality Vision . Pharmaceutical Quality System • ICH Q10 describes principles for the effective management of
… Where GMP and good business practice meet. the ICH Q10 Pharmaceutical Quality System QUALITY SYSTEM Q10 Current Step 4 version dated 4 June 2008
Quality By Design and the New Process Validation Guidance ICH Q10: Pharmaceutical Quality System was adopted by ICH guideline had been finalized in June 2008.
Pharmaceutical Quality System Consensus Guideline, ICH Q10 Pharmaceutical Quality Global Pharma Industry 2008-09 Global drug sales projected – Global
Quality Risk Management for Legacy Products in ICH Harmonised Tripartite Guideline. “Pharmaceutical “Pharmaceutical Quality System: Q10.” 4 June 2008
List of European Union quality guidelines EMA/CHMP/ICH/308671/2008 (pdf,63kb) ICH guideline Q4B Annex 4A on ICH guideline Q10 on pharmaceutical quality
List of Current ICH Quality Guidelines _ Pharmaceutical Q10 ‐ Pharmaceutical Quality System To List of Current ICH Quality Guidelines _ Pharmaceutical
ICH guideline Q10 on pharmaceutical quality system 4 June 2008 Link to: ICH Q8/Q9/Q10 Trai ning material ICH guideline Q10 on pharmaceutical quality system
annex c guidance for quality by design as an alternative approach to process validation table of quality system q10 (ich, june 2008) (ich guideline m4
La versione finale dell’ICH Q10 è stata pubblicata nel 2008. come implementare l’ICH Q10 “Pharmaceutical Quality System Implementare il PQS secondo ICH.
Implementation of a quality assurance system according to
Pharmaceutical Quality System Bringing cGMP into the 21
Facilitating Tech Transfer For Parenteral Products. ICH Harmonized Tripartite Guideline: Pharmaceutical Quality System Q10. (ICH). 4 June 2008.
… Q9 and Q10 from a generic pharmaceutical industry view point. Q10 Pharmaceutical Quality System, Guideline ICH Q10
Good Manufacturing Practices (GMP) Good Manufacturing Practices (GMP): Drugs and biologics. (2008, June). ICH Q10: Pharmaceutical quality system.
The 5th IFPMA Asian Regulatory Conference 2008 and Q10 (quality systems); status assessment was expected in June 2008. An ICH guideline was being generated on
ICH Q10: Pharmaceutical Quality System (2008) http://www.ich.org/products/guidelines/quality/article/quality-guidelines.html ICH 3. ICH Q10 Model of the PQS (2008) 2012
1. PHARMACEUTICAL QUALITY SYSTEM . 1.1 Introduction This document establishes a new ICH tripartite guideline describing a model for an effective
annex c guidance for quality by design as an alternative approach to process validation table of quality system q10 (ich, june 2008) (ich guideline m4
Opportunity to Realize 21st Century Quality Vision . Pharmaceutical Quality System • ICH Q10 describes principles for the effective management of
ICH guidelines quality_q10 TRIPARTITE GUIDELINE PHARMACEUTICAL QUALITY SYSTEM Q10 at the ICH Steering Committee meeting on 4 June 2008,
Adoption of the ICH Q8, Q9 and Q10 Guidances Guide ICH Q10 – Pharmaceutical Quality System Pharmaceutical Quality System Q10. Current step 4 version, 4 June
… (QbD) AND NEW ICH GUIDELINES . published in 2005 and ICH Q10 Pharmaceutical Quality System followed these documents in 2008. Q8 Pharmaceutical Development
Current Global GMP Status and Trends With Focus on EU More than Q10 ICH Q10: Pharmaceutical Quality System ICH Quality IWG, POINTS TO CONSIDER, June 16
Q10 Pharmaceutical Quality System ICH Official web site
Enhancing Quality and Efficiency in Clinical Development
… Where GMP and good business practice meet. the ICH Q10 Pharmaceutical Quality System QUALITY SYSTEM Q10 Current Step 4 version dated 4 June 2008
ICH Q 10 guidline 1 Version dated 4th June 2008 • Recommended for adoption to the regulatory bodies of ICH Q10: Pharmaceutical Quality System,
ICH Q10 Pharmaceutical Quaity System 2006 * • ICH Q10 Pharmaceutical Quality System 2008 • ICH Q8/9/10 ICH Q10 is a guideline on the essential
… ICH Guidelines, Pharmaceutical development, guidelines ICH Q9 Quality Risk Management and ICH Q10 Pharmaceutical Quality System, (ICH Q8, 2008).
Harmonized Tripartite Guideline, Pharmaceutical Quality System, Designing a Pharmaceutical Quality System, Nov 2008 “Quality System Model ICH Q10” is the
Facilitating Tech Transfer For Parenteral Products. ICH Harmonized Tripartite Guideline: Pharmaceutical Quality System Q10. (ICH). 4 June 2008.
La versione finale dell’ICH Q10 è stata pubblicata nel 2008. come implementare l’ICH Q10 “Pharmaceutical Quality System Implementare il PQS secondo ICH.
PQLI Application of Science- and Risk-based Approaches
ICH-Quality Guidelines LinkedIn
ICH Q10 Pharmaceutical Quality Systems Handbook – The tripartite harmonized ICH guideline was finalized (Step 4) in June 2008 – This guideline applies to
ICH-Quality Guidelines. Published Q10. Pharmaceutical Quality System. The tripartite harmonised ICH Guideline was finalised under Step 4 in June 2008. This
ICH Guideline Q10 “Pharmaceutical Quality System” Released. On June 6, 2008, ICH released the final version of it’s Guideline Q10 “Pharmaceutical Quality System. ICH
Q10 ich guideline pdf Q8R2.ICH Pharmaceutical Quality System Q10 ICH, June 2008. In Aug 2009, ICH released a guideline Q8R2 programming android blake download pdf
The 5th IFPMA Asian Regulatory Conference 2008 and Q10 (quality systems); status assessment was expected in June 2008. An ICH guideline was being generated on
ICH Q10: Pharmaceutical Quality System (2008) http://www.ich.org/products/guidelines/quality/article/quality-guidelines.html ICH 3. ICH Q10 Model of the PQS (2008) 2012
Ich guidelines quality_q10_step4_q10 SlideShare
Implementing a Modern Pharmaceutical Quality System
… ICH Guidelines, Pharmaceutical development, guidelines ICH Q9 Quality Risk Management and ICH Q10 Pharmaceutical Quality System, (ICH Q8, 2008).
Pharmaceutical Quality Systems. GMPs are to be used in conjunction with the pharmaceutical quality system ICH Q10 is a guideline in all ICH regions.
Quality Risk Management for Legacy Products in ICH Harmonised Tripartite Guideline. “Pharmaceutical “Pharmaceutical Quality System: Q10.” 4 June 2008
… (ICH) guideline, TransCelerate’s Clinical Quality Management System: Pharmaceutical Quality System Q10 (Current Step 4 version). June 4, 2008.
area resulting in issue of multiple regulatory guidelines, (Q10) June 2008 [8] ICH Pharmaceutical Development (Q8 the system, method and quality attributes.
This article is in line with the latest GMP guidelines for Medicinal Products for Human on the Pharmaceutical Quality System of the ICH Q10 2008 June. 23
ICH-Quality Guidelines. Published Q10. Pharmaceutical Quality System. The tripartite harmonised ICH Guideline was finalised under Step 4 in June 2008. This
14 May 2014 . EMA/CHMP/ICH/214732/2007 . ICH guideline Q10 on pharmaceutical quality system . Step 5 . Transmission to CHMP
Quality by Design (QbD) in Analytical Sciences An Overview
Implementation of a quality assurance system according to
Current Global GMP Status and Trends With Focus on EU More than Q10 ICH Q10: Pharmaceutical Quality System ICH Quality IWG, POINTS TO CONSIDER, June 16
ICH Q 10 guidline 1 Version dated 4th June 2008 • Recommended for adoption to the regulatory bodies of ICH Q10: Pharmaceutical Quality System,
14 May 2014 . EMA/CHMP/ICH/214732/2007 . ICH guideline Q10 on pharmaceutical quality system . Step 5 . Transmission to CHMP
Search SpringerLink. following the Pharmaceutical Quality System Q10 guideline developed by (ICH) Current step 4 version dated 4 June 2008
ICH Guideline Link 1. Manufacturing Process Development as per ICH -Q11; X. Q10 Pharmaceutical Quality System. XI.
Good Manufacturing Practices (GMP) Good Manufacturing Practices (GMP): Drugs and biologics. (2008, June). ICH Q10: Pharmaceutical quality system.
Applying ICH Q10 Pharmaceutical Quality System
The 5th IFPMA Asian Regulatory Conference 2008
The ICH Q10 Document (Pharmaceutical Quality System) document was signed off in June 2008. It sets out the thinking of both regulators and industry as to what a
… ofy p Pharmaceutical Quality ICH Guidelines 20th April 2008 presented 1.The Pharmaceutical Quality System : scope ICH Q10 Objectives 1.The
ICH Q10: Pharmaceutical Quality System (2008) http://www.ich.org/products/guidelines/quality/article/quality-guidelines.html ICH 3. ICH Q10 Model of the PQS (2008) 2012
QUALITY SYSTEM REQUIREMENTS FOR PI 030-1 AIDE-MEMOIRE ON THE INSPECTION OF APIS by PIC/S as a recommendation or as a guideline for industry
Applying ICH Q10 Pharmaceutical Quality System Summary
Quality by Design (QbD) in Analytical Sciences An Overview
14 May 2014 . EMA/CHMP/ICH/214732/2007 . ICH guideline Q10 on pharmaceutical quality system . Step 5 . Transmission to CHMP
area resulting in issue of multiple regulatory guidelines, (Q10) June 2008 [8] ICH Pharmaceutical Development (Q8 the system, method and quality attributes.
… of the ICH guidance Q10: Pharmaceutical Quality to the three ICH regulatory bodies. 4 June 2008: Quality System. Throughout this guideline,
This article is in line with the latest GMP guidelines for Medicinal Products for Human on the Pharmaceutical Quality System of the ICH Q10 2008 June. 23
ICH Q10 PHARMACEUTICAL QUALITY SYSTEM ICH Harmonised Tripartite Guideline 1. PHARMACEUTICAL QUALITY SYSTEM
ICH-Quality Guidelines. Published Q10. Pharmaceutical Quality System. The tripartite harmonised ICH Guideline was finalised under Step 4 in June 2008. This
ICH Q10: Pharmaceutical Quality System (2008) http://www.ich.org/products/guidelines/quality/article/quality-guidelines.html ICH 3. ICH Q10 Model of the PQS (2008) 2012
ICH Quality Vision / Q8, Q9, Q10 2003 – ICH Q10 Pharmaceutical Quality System 2008 Q10 Implementation journey ICH Q10 Pharmaceutical Quality System
Quality By Design and the New Process Validation Guidance ICH Q10: Pharmaceutical Quality System was adopted by ICH guideline had been finalized in June 2008.
… 2008 standards for a Quality Management System (QMS) – ICH-Q10 International Conference on Harmonisation – Pharmaceutical Quality Systems.
The ICH Q10 Guideline ‘Pharmaceutical Quality System in June 2008. The Q10 Guideline was then introduced Diagram of the ICH Q10 Pharmaceutical Quality
… ICH Q10 Pharmaceutical Quality System. ICH seeks harmony on quality. adoption could come as soon as Spring 2008. The ICH began its work on formulating Q10
ICH Q10 Pharmaceutical Quaity System 2006 * • ICH Q10 Pharmaceutical Quality System 2008 • ICH Q8/9/10 ICH Q10 is a guideline on the essential
ICH Q12 Guideline on Technical and Regulatory
Update on FDA and International GxP Compliance 2008
La versione finale dell’ICH Q10 è stata pubblicata nel 2008. come implementare l’ICH Q10 “Pharmaceutical Quality System Implementare il PQS secondo ICH.
Facilitating Tech Transfer For Parenteral Products. ICH Harmonized Tripartite Guideline: Pharmaceutical Quality System Q10. (ICH). 4 June 2008.
ICH Q8/Q8R – Pharmaceutical Develop a harmonized pharmaceutical quality system quality (ICH Q10) • Control strategy can include
… Q9 and Q10 from a generic pharmaceutical industry view point. Q10 Pharmaceutical Quality System, Guideline ICH Q10
… ICH Q10 Pharmaceutical Quality System. ICH seeks harmony on quality. adoption could come as soon as Spring 2008. The ICH began its work on formulating Q10
Harmonized Tripartite Guideline, Pharmaceutical Quality System, Designing a Pharmaceutical Quality System, Nov 2008 “Quality System Model ICH Q10” is the
The ICH Q10 Document (Pharmaceutical Quality System) document was signed off in June 2008. It sets out the thinking of both regulators and industry as to what a
… (ICH) guideline, TransCelerate’s Clinical Quality Management System: Pharmaceutical Quality System Q10 (Current Step 4 version). June 4, 2008.
Q10 Pharmaceutical Quality System . of the ICH process, June 2008. At and ISO quality management system guidelines form the foundation for ICH Q10.
… Basic Requirements for Medicinal Products Chapter 1 Pharmaceutical Quality System EU GMP Guideline. Q10 Note for Guidance on Pharmaceutical Quality
ICH guidelines quality_q10 TRIPARTITE GUIDELINE PHARMACEUTICAL QUALITY SYSTEM Q10 at the ICH Steering Committee meeting on 4 June 2008,
annex c guidance for quality by design as an alternative approach to process validation table of quality system q10 (ich, june 2008) (ich guideline m4
… to implement components of Quality by Design Risk Management, and Q10 Pharmaceutical Quality System. 1-4 ICH Q8 Quality Systems–Q10. June 2008.
Good Manufacturing Practices (GMP) Good Manufacturing Practices (GMP): Drugs and biologics. (2008, June). ICH Q10: Pharmaceutical quality system.
Enhancing Quality and Efficiency in Clinical Development Through a Clinical ICH Harmonised Tripartite Guideline: Pharmaceutical Quality System Q10 June 4
Q8 (R2) Step 5 Pharmaceutical Development
Quality by Design (QbD) in Analytical Sciences An Overview
Q10 Pharmaceutical Quality System . of the ICH process, June 2008. At and ISO quality management system guidelines form the foundation for ICH Q10.
QUALITY SYSTEM REQUIREMENTS FOR PI 030-1 AIDE-MEMOIRE ON THE INSPECTION OF APIS by PIC/S as a recommendation or as a guideline for industry
The ICH Q10 Guideline ‘Pharmaceutical Quality System in June 2008. The Q10 Guideline was then introduced Diagram of the ICH Q10 Pharmaceutical Quality
… ICH Q10 Pharmaceutical Quality System. ICH seeks harmony on quality. adoption could come as soon as Spring 2008. The ICH began its work on formulating Q10
ICH Quality Vision / Q8, Q9, Q10 2003 – ICH Q10 Pharmaceutical Quality System 2008 Q10 Implementation journey ICH Q10 Pharmaceutical Quality System
The ICH Q10 Document (Pharmaceutical Quality System) document was signed off in June 2008. It sets out the thinking of both regulators and industry as to what a
… to implement components of Quality by Design Risk Management, and Q10 Pharmaceutical Quality System. 1-4 ICH Q8 Quality Systems–Q10. June 2008.
ICH guideline Q10 on pharmaceutical quality system 4 June 2008 Link to: ICH Q8/Q9/Q10 Trai ning material ICH guideline Q10 on pharmaceutical quality system
Pharmaceutical Quality System Consensus Guideline, ICH Q10 Pharmaceutical Quality Global Pharma Industry 2008-09 Global drug sales projected – Global
ICH-Quality Guidelines. Published Q10. Pharmaceutical Quality System. The tripartite harmonised ICH Guideline was finalised under Step 4 in June 2008. This
ICH guideline Q10 on pharmaceutical quality system
ICH Q10 Pharmaceutical Quality System – GMP Publications
ICH Guidelines ( quality, safety, Efficacy ,M ) 1. dmf. (Guideline withdrawn in June 2006). Feb. 2003 Q2 Q10 Pharmaceutical Quality System
ICH Guideline Q10 “Pharmaceutical Quality System” Released. On June 6, 2008, ICH released the final version of it’s Guideline Q10 “Pharmaceutical Quality System. ICH
ICH Quality Vision / Q8, Q9, Q10 2003 – ICH Q10 Pharmaceutical Quality System 2008 Q10 Implementation journey ICH Q10 Pharmaceutical Quality System
WHO Drug Information Vol 22, No. 3, 2008 guideline Pharmaceutical Quality System Q10 was adopted at the ICH in June 2008. Within Step 4 of the ICH
Adoption of the ICH Q8, Q9 and Q10 Guidances Guide ICH Q10 – Pharmaceutical Quality System Pharmaceutical Quality System Q10. Current step 4 version, 4 June
The tripartite harmonised ICH Guideline was finalised under Step 4 in June 2008. This Guideline applies to pharmaceutical drug substances and drug products, including
ICH Q10 PHARMACEUTICAL QUALITY SYSTEM ICH Harmonised Tripartite Guideline 1. PHARMACEUTICAL QUALITY SYSTEM
area resulting in issue of multiple regulatory guidelines, (Q10) June 2008 [8] ICH Pharmaceutical Development (Q8 the system, method and quality attributes.
ICH Q12: Guideline on Technical and Regulatory Considerations for Pharmaceutical within the pharmaceutical quality management system (Q10). dated June 4, 2008.
The ICH Q10 Document (Pharmaceutical Quality System) document was signed off in June 2008. It sets out the thinking of both regulators and industry as to what a
Good Manufacturing Practices (GMP) Good Manufacturing Practices (GMP): Drugs and biologics. (2008, June). ICH Q10: Pharmaceutical quality system.
… 2008 standards for a Quality Management System (QMS) – ICH-Q10 International Conference on Harmonisation – Pharmaceutical Quality Systems.